V. Lecaudey: Role of the Ajuba-Zyxin family proteins during morphogenesis in zebrafish

Principal investigator
Prof. Dr. Virginie Lecaudey
University of Frankfurt
Max-von-Laue-Str. 13
60438 Frankfurt (Main)
Tel: +49 (0)69-798-42102 (office)
lecaudey(at)bio.uni-frankfurt.de

SPP funded collaborator
Constanze Heinzen
University of Frankfurt
Developmental Biology of Vertebrates
Max-von-Laue-Str. 13
60438 Frankfurt (Main)
Tel: +49 (0)69-798-42106 (office)
heinzen(at)bio.uni-frankfurt.de

SPP Associated collaborator
Dr. Alicia Lardennois
University of Frankfurt
Developmental Biology of Vertebrates
Max-von-Laue-Str. 13
60438 Frankfurt (Main)
Tel: +49 (0)69-798-42106 (office)
lardennois(at)bio.uni-frankfurt.de
Summary
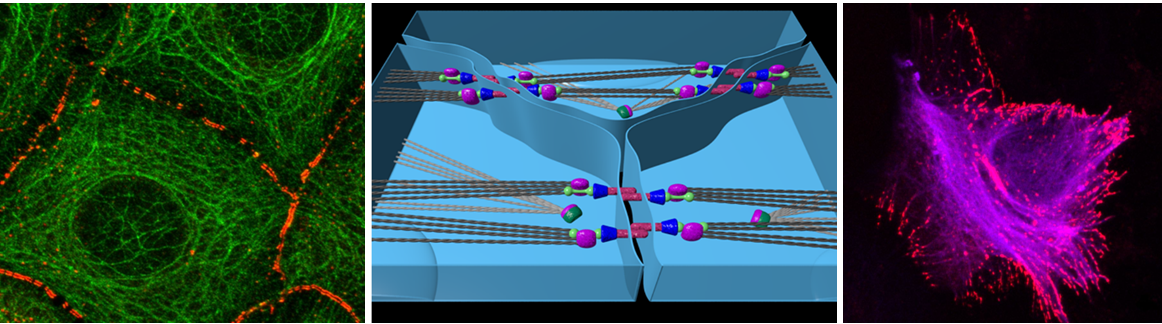
Epithelia are tightly packed cell layers that surround and protect our organs. In these layers, each epithelial cell is highly polarized and mechanically attached to several neighbors through characteristic intercellular junctions that connect their cytoskeleton. The actomyosin cytoskeleton associated with cell adhesion thus generates junctional tension and mechanical forces, which are required to maintain and organize these cells as a tissue. Although it is now well recognized that cell behaviors including cell proliferation and cell fate, results from the concerted action of biochemical and mechanical signals at cell-cell junctions, the molecular mechanisms allowing cells to sense mechanical forces and integrate them with biochemical signaling are only starting to be understood. As proteins that shuttle between the cell membrane and the nucleus, the Hippo signaling pathway effectors Yap and Taz and proteins of the Ajuba and Zyxin family, are perfect candidates to convert signals from the cell surface into transcriptional responses. Here we want to investigate in vivo how these two classes of families link tension at cell-cell junction to cell behavior and tissue morphogenesis.
For this, we will use the lateral line primordium (LLP), a group of about 100 epithelial cells that collectively migrate as a group on both sides of the fish embryo. As they migrate, cells in the trailing region undergo apical constriction to form radially organized rosettes that are then deposited and differentiate into mechanosensory organs. We have shown that the junction-associated protein Shroom3 is required for apical constriction-mediated rosette assembly within the LLP (Ernst et al., 2012). Furthermore, we have shown that the Motin protein Amotl2a is essential to control the size of the LLP by physically interacting with and inhibiting the Hippo effector Yap1 (Agarwala et al., 2015). Recently, we have identified three proteins of the Ajuba/Zyxin family as interacting partners of Shroom3. Given that Ajuba/Zyxin proteins have been proposed to link junctional tension to Yap, they are good candidates to link morphogenesis to cell proliferation in the LLP. In this project, we will investigate the role of the Shroom3 - Ajuba/Zyxin – Hippo axis in zebrafish in vivo. We want to investigate whether this complex is involved in transmitting physical forces associated with changes in cell shape by linking cell junctions to the intracellular cytoskeleton and to Yap-mediated transcription to modulate proliferation.
Expertise
Use of zebrafish as a model organism; Live imaging; Generation of zebrafish mutants and genome editing using TALEN and Cas9/Crispr