K. Breuhahn: Connecting intercellular cell junctions with Hippo/YAP pathway activity in hepatocytes and liver tumor cells

Principal investigator
Dr. Kai Breuhahn
Universitätsklinikum Heidelberg
Pathologisches Institut
Im Neuenheimer Feld 224
69120 Heidelberg
Tel: +49 (0)6221-56-4675 (office)
kai.breuhahn(at)med.uni-heidelberg.de

SPP funded collaborator
Lena Thiess
Universitätsklinikum Heidelberg
Pathologisches Institut
Im Neuenheimer Feld 224
69120 Heidelberg
lena.thiess(at)med.uni-heidelberg.de
Summary
The cell density-sensing Hippo pathway is an evolutionary conserved signalling cascade regulating regenerative processes and organ size. Its comprises a central Hippo kinase cassette consisting of the serine/threonine kinases Lats1/2 and Mst1/2, which upon activation, phosphorylate the transcriptional cofactor yes-associated protein (YAP). Hyper-phosphorylation of YAP is leading to the cytoplasmic retention and degradation of YAP. In contrast, inactivation of the Hippo pathway induces the accumulation of hypo-phosphorylated YAP in the nucleus, where it interacts with different transcription factors (TFs; e.g. TEAD1-4, FoxM1) to drive the expression of pro-proliferative and anti-apoptotic target genes. The organ growth controlling properties of the Hippo/YAP axis under physiological conditions was illustrated by genetic mosaic screens in Drosophila melanogaster and genetically modifies mouse models. Here, the inactivation of the Hippo kinase cassette or activation of YAP (or its orthologue Yorkie in D. melanogaster) are leading to tissue overgrowth and organ hyperplasia. Under pathophysiological conditions the Hippo pathway contributes to tumour initiation in several organs such as liver cancer. Interestingly, no distinct ligand/receptor combination is regulating Hippo/YAP activity. Instead, its ability to sense extracellular information and cell-cell contact is a central modifier of Hippo activity and YAP localization.
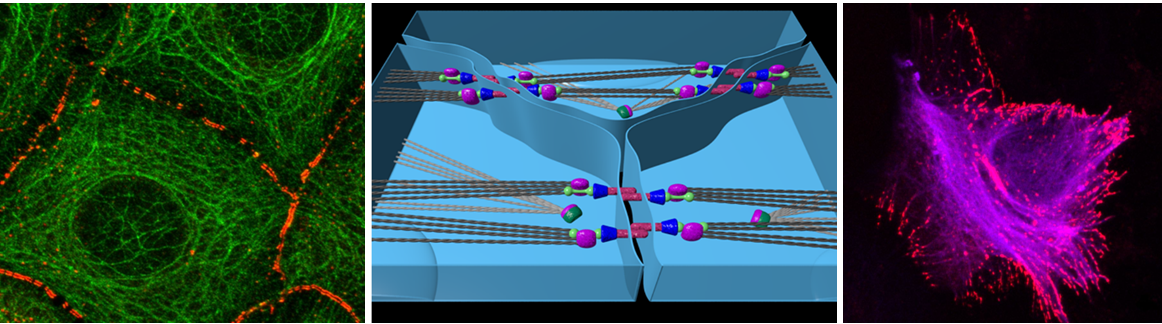
Cellular/structural components, such as adherens junctions (AJs), cytoskeletal polymerisation status, and cell polarity complexes connect extracellular information with the Hippo/YAP pathway. Interestingly, not any kind of hepatocellular polarity disturbance affects YAP activity, clearly indicating the existence defined molecular mechanisms linking sensors of cell-cell contact and 3-dimensional orientation, with the biological properties of the Hippo pathway. In this context, first data illustrate that cadherin family members represent key hubs for the integration of extracellular information into the Hippo pathway. For example, the atypical cadherin FAT1 in D. melanogaster and the classical E-cadherin in mammalian cells mediate contact inhibition through the Hippo pathway. Because FAT1 and E-cadherin significantly differ in their structure and biological properties, an impact of other (so-far not identified) cadherin family members on the Hippo/YAP axis is highly suggestive.
Together, these results strongly suggest a direct link between AJs integrity as well as the presence (or absence) of cadherin family members with properties of the Hippo/YAP pathway under physiological and pathophysiological conditions. However, a detailed understanding of how AJs or desmosomal cadherins facilitate their regulatory properties in a Hippo/YAP-dependent manner is not understood.
Expertise
Our group is focussing on the oncogenic properties of the Hippo/YAP pathway in liver tumorigenesis. For this we make use suitable in vitro systems (immortalized and primary cells derived from liver and cancerous tissues), human samples (e.g. premalignant and malignant liver tissues), and in vivo model systems (e.g. liver cell-specific overexpression as well as gene silencing).