A Diz-Muñoz: Deciphering the role of mechanics for epithelia trans-differentiation

Principal investigator

SPP funded collaborator
Summary
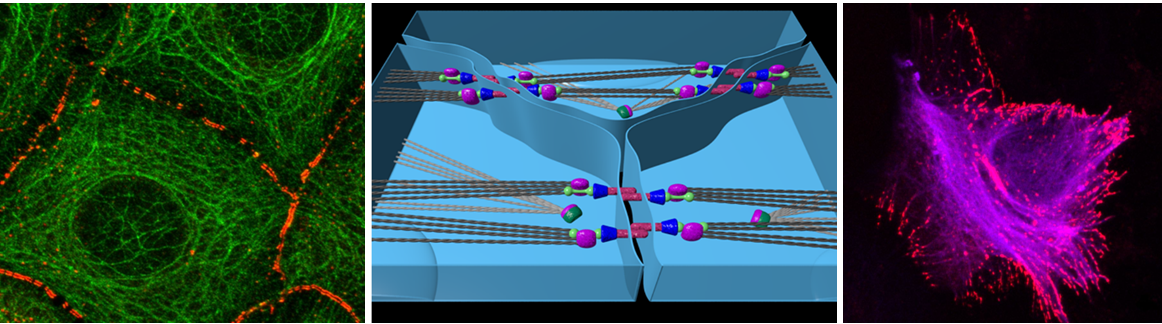
In the last decade it has become clear that cellular and microenvironment mechanics affect epithelia integrity and cell fate. Upon mechanical injury of the zebrafish notochord, cell fate plasticity occurs as epithelial sheath cells trans-differentiate into non-epithelial vacuolated ones. For sheath tissue integrity, such trans-differentiation requires the coordination of the cell’s cytoskeleton with a concomitant rearrangement of their cell-cell junctions. A clear understanding of the molecular and physical mechanisms governing cell trans-differentiation in vivo have been hampered by the lack of convenient assay systems and tools to assess and perturb mechanical properties in 3D environments. In this proposal, we will first characterize cell-cell junctions in sheath cells by light and electron microscopy, while quantifying tissue stiffness by Brillouin microscopy upon controlled vacuolated cell death (Objective 1). Next, we will perturb the balance of forces between neighboring sheath cells by developing strategies to systematically change tissue stiffness, neighboring cell contractility and adhesion, while assessing their effect on fate and cell-cell junctions’ dynamics during epithelia trans-differentiation (Objective 2).
Expertise
Surface mechanics, Atomic force microscopy, Brillouin microscopy, zebrafish development, cell identity