N. Schlegel, J. Waschke: Role of Dsg2-dependent adhesion and signalling in Crohn’s disease

Principal investigator
Prof. Dr. Nicolas Schlegel
Universität Würzburg
Klinik und Poliklinik für Allgemein-, Viszeral-, Gefäß-, und Kinderchirurgie
schlegel_n(at)ukw.de

Principal investigator
Prof. Dr. Jens Waschke
Ludwig-Maximilians-Universität München
Institute of Anatomy and Cell Biology
Department I
Pettenkoferstraße 11
80336 München
jens.waschke(at)med.uni-muenchen.de

SPP funded collaborator
Hanna Ungewiss
Ludwig-Maximilians-Universität München
Institute of Anatomy and Cell Biology
Department I
Pettenkoferstraße 11
80336 München
hanna.ungewiss(at)med.uni-muenchen.de

SPP funded collaborator
Jessica Neubauer
Ludwig-Maximilians-Universität München
Institute of Anatomy and Cell Biology
Pettenkoferstraße 11
80336 München
Tel: +49 (0)89-2180-72623 (office)
jessica.neubauer(at)med.uni-muenchen.de
Summary
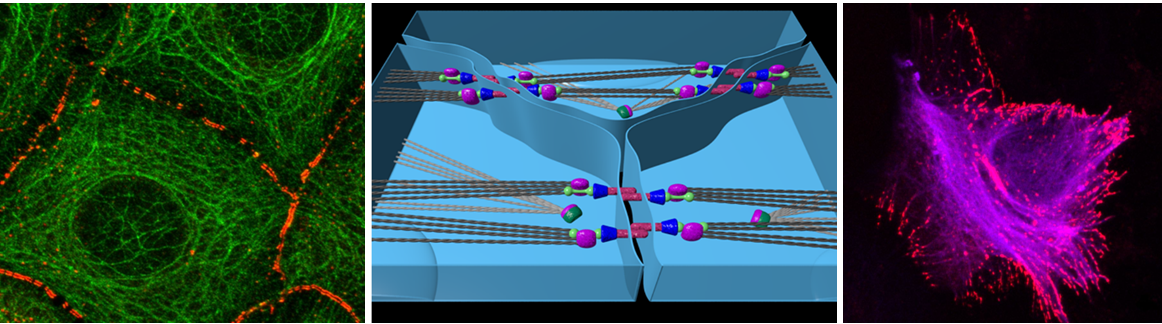
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) with complex pathogenesis which is characterized by impaired intestinal epithelial barrier integrity. We previously showed that desmosomes besides of adherens junctions are required for maintenance of barrier properties in enterocytes and that desmoglein 2 (Dsg2), which together with desmocollin 2 is the major desmosomal adhesion molecule, is crucial in this context.
Therefore, we started to investigate the role of Dsg2 in CD and found that in patients’ intestinal biopsies suffering from conservative refractory CD Dsg2 is reduced and displays altered localization. A possible role of Dsg2 in CD is new and the mechanisms by which Dsg2 strengthens the intestinal barrier are unclear. As with other autoimmune diseases treatment options in CD are limited and associated with severe side-effects. Understanding the mechanism how Dsg2 is involved in CD pathogenesis would possibly allow to establish new therapeutic approaches. Preliminary data indicate that tumor necrosis factor α (TNFα), which is well known to be critically involved in CD pathogenesis compromised Dsg2-dependent enterocyte cohesion. In keratinocytes, we recently found that the contribution of desmosomal cadherins to overall cell cohesion as well as to the signalling pathways regulated by specific Dsg isoforms substantially differ and that adhesive properties and signal transduction appear to be linked. Thus, the role of Dsg2 as adhesion-dependent signaling hub in enterocytes needs to be clarified. We will investigate adhesion- and signaling-dependent functions of Dsg2 in CD in vitro and in vivo. In patients’ biopsies alterations in Dsg2 turnover and localization will be correlated with disease stage and localization within the gut. In addition, the signaling pathways by which TNFα regulates enterocyte desmosomal adhesion and which downstream of Dsg 2 control barrier properties and more specifically tight junction integrity will be determined. Therefore, in cultured monolayers of intestinal epithelial cells the effects of TNFα on Dsg2 localization and Dsg2-mediated cell cohesion will be studied using dissociation assays, atomic force microscopy as well as laser tweezers and modulated by Dsg-specific peptides. Transepithelial resistance (TER) and measurements of FITC-dextran flux across monolayers will be used to assess barrier function in vitro and DSS colitis and permeability measurements in isolated gut segments will be applied in vivo. Signaling pathways will be investigated with focus on p38MAPK, cAMP and Rho-GTPase signaling which are known to be linked to regulation of the intestinal barrier and desmosomal adhesion. Finally, the modulating effect of cortisol on Dsg2 adhesive function and on signalling pathways will be characterized.
Expertise
Regulation of desmosomes and adherens junctions in context with epithelial and endothelial barriers, force measurements on living cells by AFM