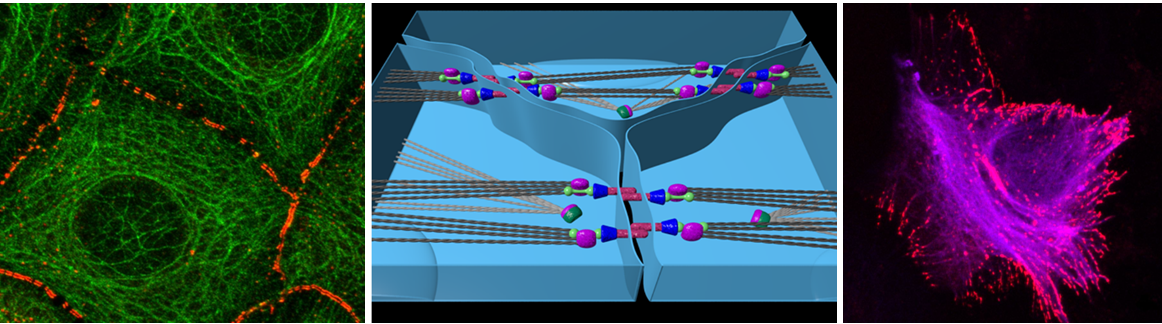
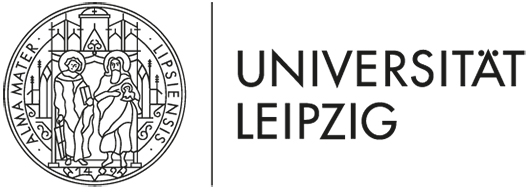J. Großhans, F. Wolf: Coordinated dynamics of epithelial cells in the amnioserosa of Drosophila embryos
Principal investigator
Prof. Dr. Jörg Großhans
Philipps-Universität Marburg
FB17/Biologie
Karl-von-Frisch-Straße 8
35032 Marburg
Tel: +49 (0)6421-282-1502 (office)
joerg.grosshans(at)biologie.uni-marburg.de
Principal investigator
Prof. Dr. Fred Wolf
Max-Planck-Institut für Dynamik und Selbstorganisation
Theoretische Neurophysik
Am Fassberg 17
37077 Göttingen
Tel: +49 (0)551-5176-423 (office)
fred(at)nld.ds.mpg.de
SPP funded collaborator
Prachi Richa
Georg-August-Universität Göttingen
Institute of Developmental Biochemistry
Justus-von-Liebig Weg 11
37077 Göttingen
Tel: +49 (0)551-398273 (office)
prachi.richa(at)med.uni-goettingen.de

SPP funded collaborator
Ankit Choudhury
Philipps-Universität Marburg
FB17/Biologie
Karl-von-Frisch-Straße 8
35032 Marburg

SPP funded collaborator
Matthias Häring
Max-Planck-Institut für Dynamik und Selbstorganisation
Theoretische Neurophysik
Am Fassberg 17
37077 Göttingen
Tel: +49 (0)551-5176-421 (office)
matthias.haering(at)ds.mpg.de
Summary
Epithelial cells are capable of sensing and reacting to forces and movements generated by or transmitted through their neighbors. Dorsal closure is a major morphogenetic transformation in Drosophila embryos that critically depends on the dynamics of a squamous epithelium called the amnioserosa. Recent findings identified the dynamics of epithelial cells in the amnioserosa (AS cells) as a promising model system for dissecting how epithelial cells coordinate their mechanical activities. AS cells (i) exhibit mechanical behaviors that depend on tissue state and appear coordinated between cells, (ii) are highly accessible to genetic intervention and quantitative live cell imaging, and (iii) exhibit coordinated activity that is statistically invariant over extended periods of time, a fundamental mathematical condition of applicability for data-driven stochastic modelling techniques. Our preliminary data indicate that in xit mutant embryos, in which E-cadherin clusters appear abnormally mobile, intercellular coordination is profoundly disturbed. This suggests that mechano-transduction by E-cadherin based signaling complexes is critical for intercellular coordination. Based on these results and taking advantage of the accessibility of the AS, the current project aims to identify and quantitatively model mechano-transduction mechanisms operating at intercellular junctions between AS cells by combining fly developmental genetics, and in vivo time-lapse imaging (JG), with methods from the mathematical theory of stochastic dynamical systems and large-scale image analysis (FW). In particular, we aim (1) to identify the molecular basis of mechano-transduction mechanisms that turn the mechanical activity and state of neighboring cells into intracellular chemical signals, (2) to use massive live-imaging data to determine quantitative models for the encoding of mechanical stimuli by intracellular chemical signals, and (3) to probe the function of AS cell mechano-transduction by genetically and optically perturbing AS cell dynamics and transduction machinery. We expect that these studies will reveal key molecular components of mechano-transduction in epithelial cells and elucidate the principles by which this machinery guides their active mechanical behavior. Methodologically, the project is designed to demonstrate and optimize a widely applicable approach for obtaining quantitative models of cellular mechano-transduction from live cell imaging in intact tissues.
Expertise
- Live imaging of embryonic development in Drosophila embryo
- Laser ablations
- Ca+2 uncaging using UV laser